Method Validation and Quality Assurance Software
Quickly analyze your method evaluation data with Abacus 3.0. Trust in our software for accurate and reliable results in your analytical method validation. With over 20 years of experience, our team is dedicated to delivering professional, compliant software to meet the needs of medical laboratories. Our Microsoft Excel add-in satisfies the stringent standards of DIN EN ISO 15189, DIN/IEC 17025, IVDR, CAP and CLSI.
Streamline your analytical method validation process with Abacus 3.0, the proven Microsoft Excel add-in for method validation. This software is used by medical laboratories, hospital laboratories, medical practices, pharmaceutical companies and IVD manufacturers worldwide. Developed by a renowned clinical pathologist, Abacus 3.0 is still under his constant expert guidance. Experience its power today.
Maximize Your Benefits with Abacus 3.0 - The Ultimate Solution for Your Method Validation Needs
Perform regulatory-compliant method evaluations using MS Excel
Comprehensive test menu for validation and verification of test procedures, quality assurance, measurement uncertainty and statistics all in one software package
Method evaluation according to ISO 15189
Meets the requirements of DIN EN ISO 15189, DIN EN ISO / IEC 17025, as well as CLSI guidelines for the verification and validation of analytical methods in the laboratory.
Validation and verification according to the CAP standard
Meets all requirements of the College of American Pathologists "All Common Checklist" for Validation and Verification of Test Procedures in the Clinical Laboratory.
Calculate Measurement Uncertainty according to ISO 15189
With Abacus 3.0 you can easily calculate and document the measurement uncertainty of your laboratory tests.
IVDR-compliant validation of Laboratory Developed Tests (LDT)
Use CLSI's evaluation protocols (EP protocols), the standard in the IVD industry, for IVDR-compliant validation of your laboratory developed tests (LDTs).
Scientifically sound
Current guidelines from CLSI, CAP, DAkkS, ISO, DIN and EMA (European Medicines Agency) are used in our statistical software for method evaluations.
Validated method validation software
The software was formally validated against the risk-based industry standard "GAMP-5 (Good Automated Manufacturing Practice)" and is therefore suitable for use in the regulated medical laboratory environment.
Seamless integration in Excel
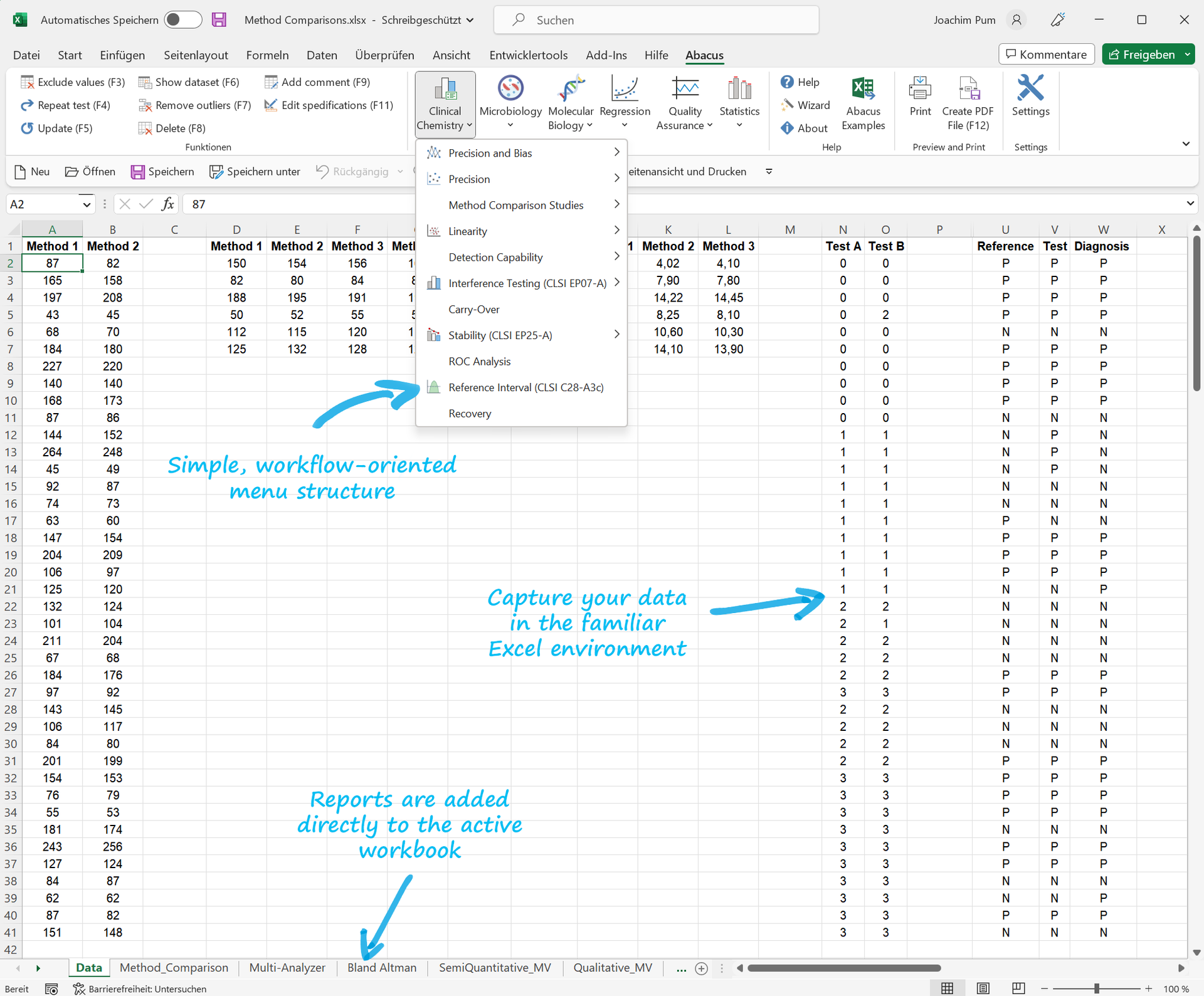
Abacus 3.0, our professional method validation software, integrates perfectly with Microsoft Excel, allowing you to manage your data in a familiar environment.
Comprehensive range of training courses
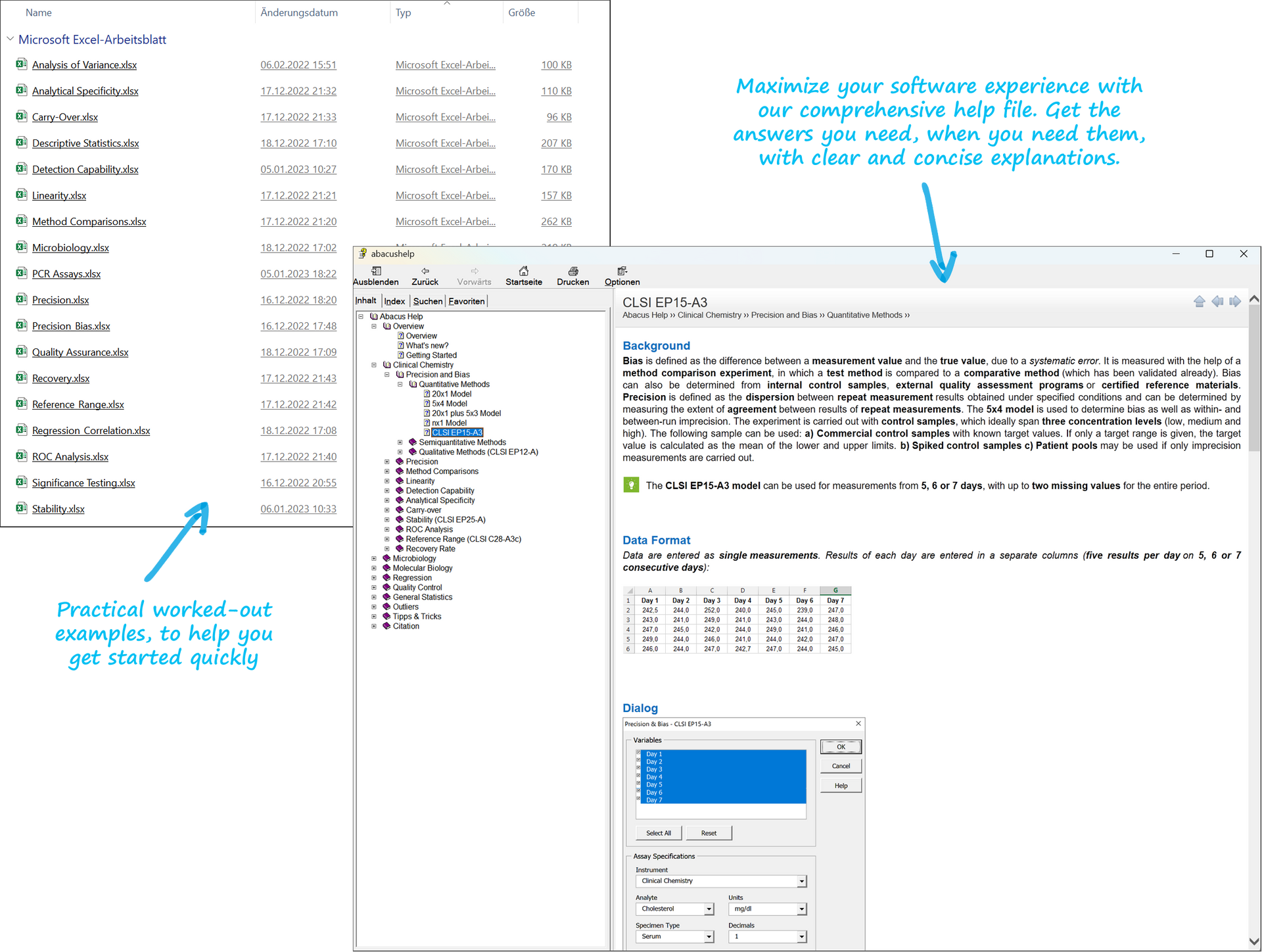
We offer a large range of training courses on topics relating to the verification and validation of test methods and laboratory statistics.
Method Validation, Lab Quality Assurance, Measurement uncertainty
Abacus 3.0, our method validation, lab quality assurance and laboratory statistics software, focuses on advanced statistical analysis of your validation and quality assurance data. The software extends the functionality of MS Excel with a series of statistical modules in the following categories:
- Regulatory- and IVDR-compliant method validation of clinical chemistry, microbiology, infectious disease serology and molecular biology assays.
- Quality assurance (including Extended Measurement Uncertainty and Youden plots for external quality assurance testing)
- Statistical functions for scientific questions
All statistical protocols are based on current recommendations from relevant professional societies and expert groups (e.g. CLSI, IFCC, CAP, FDA, ICH, EURACHEM and DIN) and recognised publications.
Validation and Verification According to Industry Standards and the IVDR
Simplify your method validation and quality assurance with Abacus 3.0. Reports are automatically formatted with your logo and institutional information, ready for printing. Our software meets all regulatory requirements for laboratory developed test ("LDT") method validation, CE-certified/FDA-approved procedure verification, and internal quality assurance. Validated against GAMP-5 and compliant with CFR Part 11, Abacus 3.0 is the ideal solution for regulated medical labs.
Streamline Your Method Validation Process with Easy Onboarding
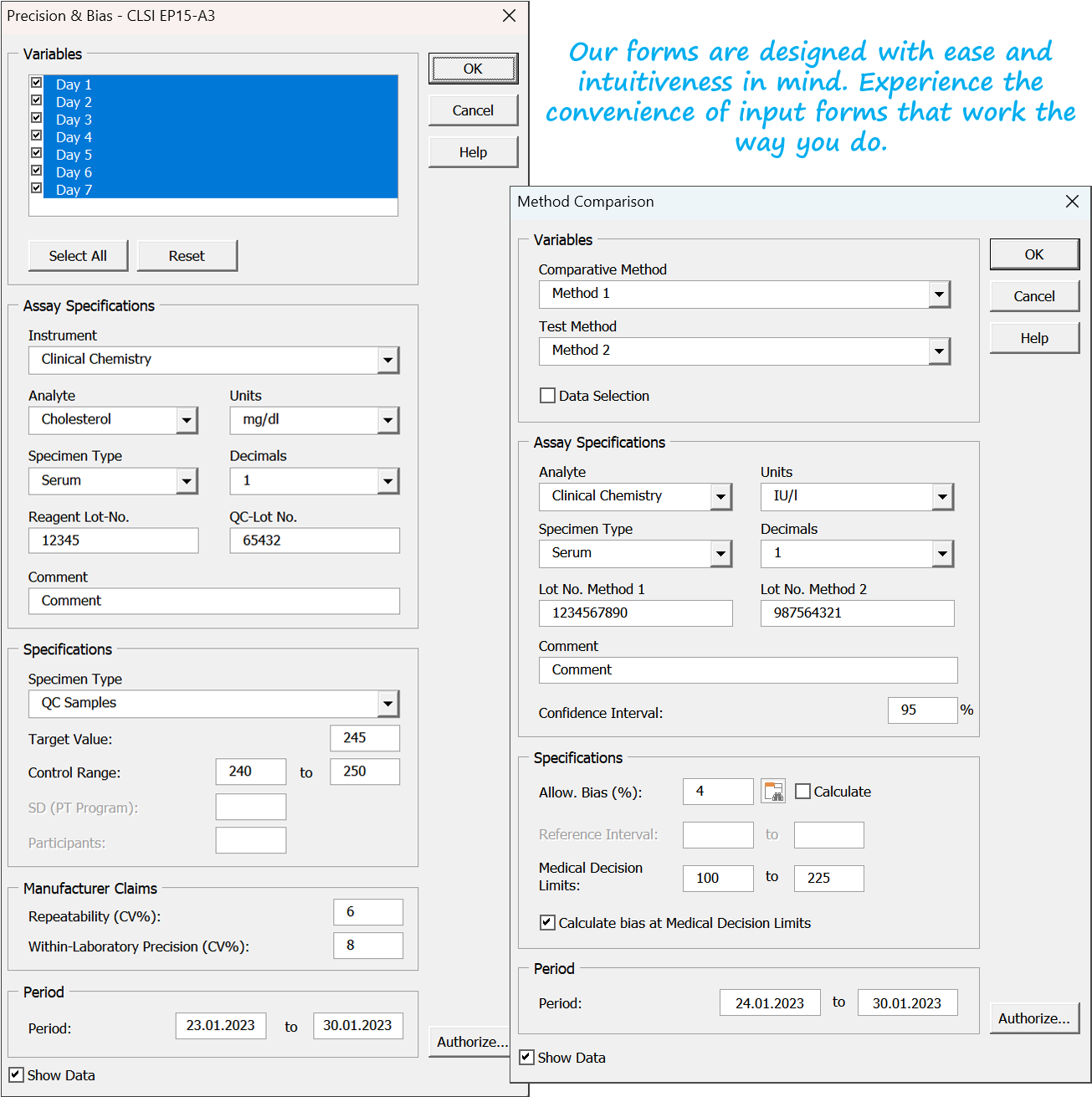
Easily launch into method validation and quality assurance with Abacus 3.0. Our software offers a quick and reliable solution for method verification of CE-certified/FDA-approved methods according to ISO 15189 or complete validation of LDTs and modified methods to meet IVDR requirements. Get started with ease thanks to detailed documentation, sample files, and a user-friendly data entry wizard. Enjoy a user-friendly interface, reliability, and workflow-oriented menu structure. Generate regulatory-compliant reports for your method validation and quality assurance in Microsoft Excel with our add-in."
The Perfect Tool for Validation of Laboratory Developed Tests (LDTs)
Ensure top-notch compliance for your lab with ISO 15189, ISO/IEC 17025, and IVDR for LDTs using Abacus 3.0, our state-of-the-art method validation & verification software. Effortlessly calculate accuracy, precision, limits of detection/quantitation, reference intervals, linearity, and more with just a few clicks. Abacus 3.0 provides a comprehensive database for evaluating validation and quality assurance data, allowing for easy adoption of precision and accuracy limits based on scientific recommendations. Customize and integrate your lab's limits with ease using our user-friendly, editable interface.
Find Expert Answers for Your Method Validation Needs - Contact Us for More Information on Our Software and Company
Contact
Phone. : +49 (0)3641 8989850
Fax: +49 (0)3641 2414927
info@lab-analytics.com
Opening hours
Monday - Friday: 9 a.m. - 5 p.m.
Saturday - Sunday: Closed
All rights reserved | LABanalytics GmbH